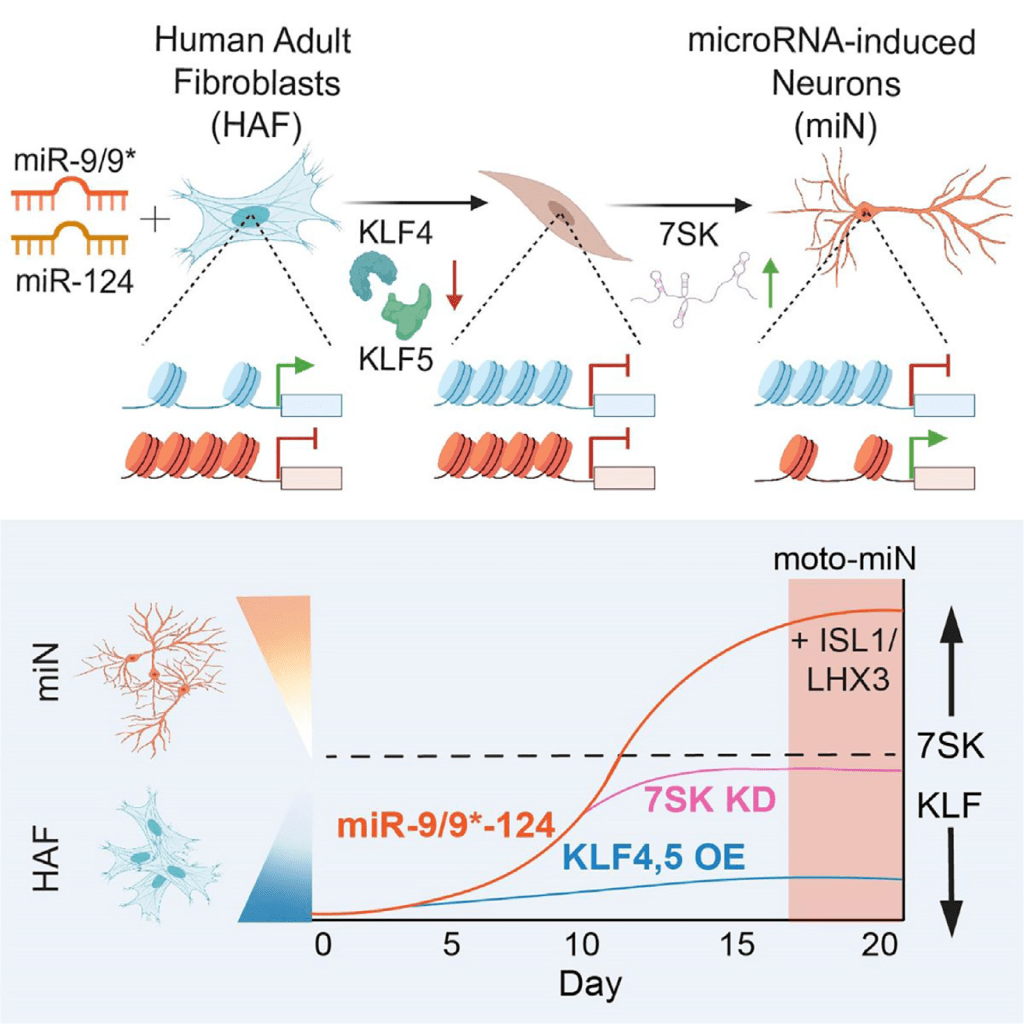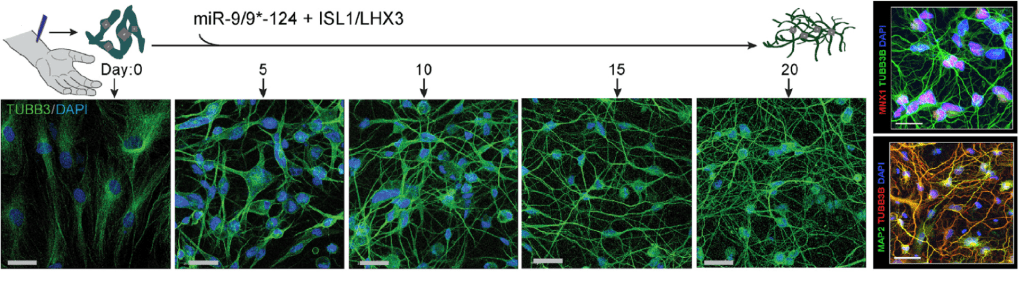
The general goal of our research is to understand genetic and molecular mechanisms underlying neurogenesis and devise cellular reprogramming approaches to generate human neurons and model late-onset neurodegenerative disorders. Our work centers around two brain-enriched microRNAs, miR-9/9* and miR-124 (miR-9/9*-124) which we identified as potent neurogenic molecules that evoke direct conversion (reprogramming) of human adult fibroblasts into neurons when ectopically expressed. These directly reprogrammed neurons retain the age of fibroblasts donors, thereby allowing us to assess neuronal properties inherent in adult neurons.
We currently aim to:
1) devise cell-fate reprogramming strategies to generate human neurons of specific subtypes by directly converting human skin fibroblasts. Our reprogramming paradigm is based on the neurogenic cellular state provided by miR-9/9* and miR-124, and transcription factors that guide the neuronal conversion towards specific subtypes.
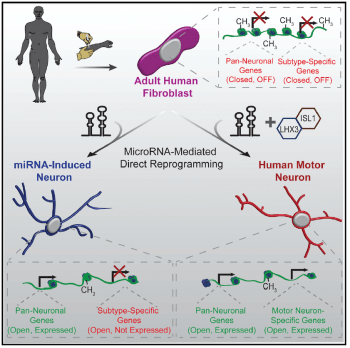
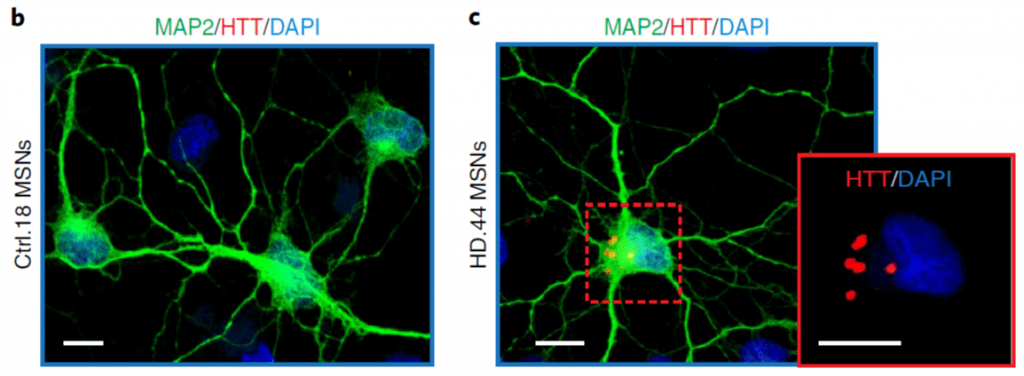
2) develop cellular models of neurological diseases using patient-derived neurons to study cell-autonomous properties associated with the disease state in induced human neurons. We are currently developing human cell culture models of neuronal aging, Huntington’s disease, and Alzheimer’s disease using patient-specific neurons generated by neuronal reprogramming.
3) identify molecular mechanisms underlying the neurogenic activity of miR-9/9* and miR-124. We use interdisciplinary approaches (molecular genetics, genomics, and biochemistry) to delineate how these microRNAs promote neuronal fate. Specifically, we are interested in understanding changes in chromatin remodeling complex activities and changes in chromatin landscape mediated by miR-9/9* and miR-124.